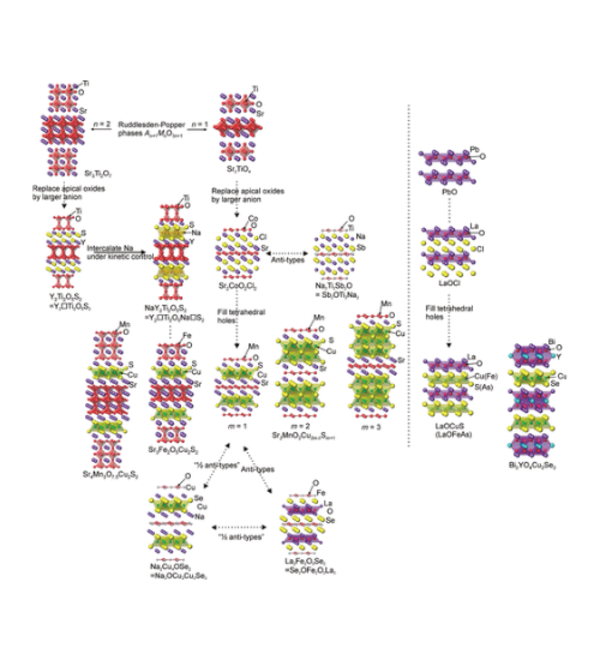
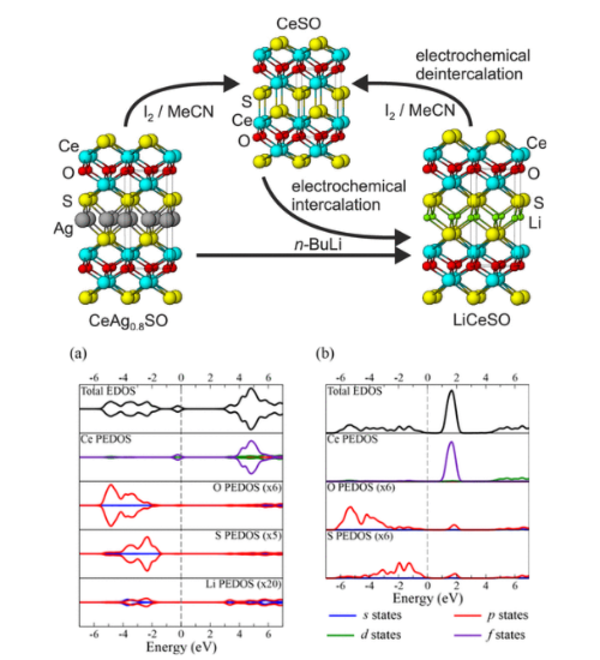
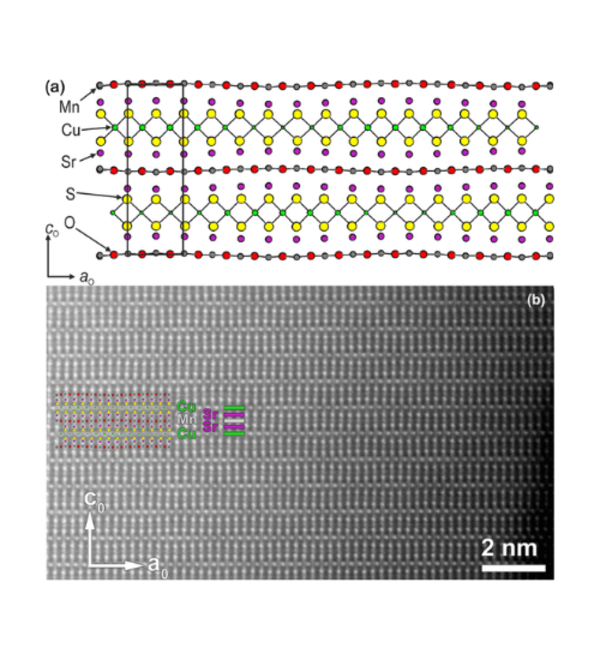
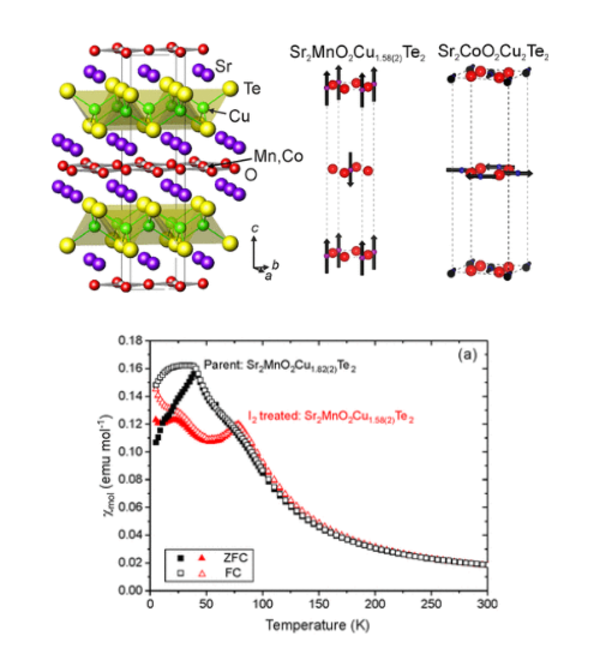
Layered Oxychalcogenides
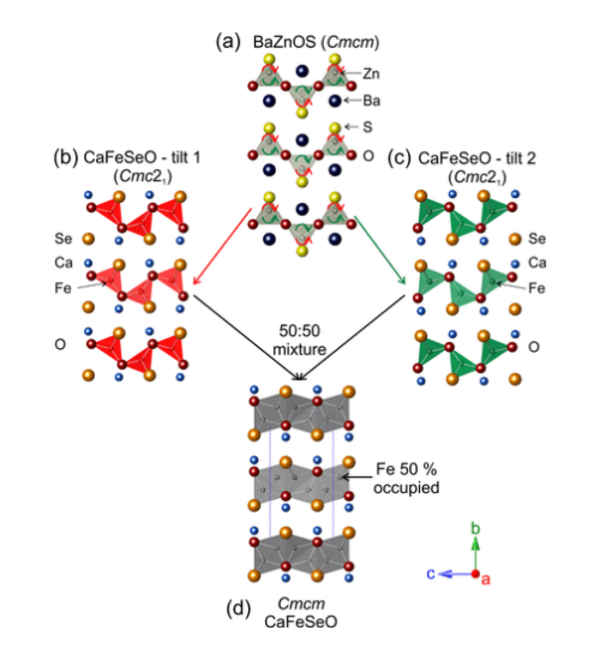
Much of the work in the group focuses on layered oxide chalcogenide and oxide pnictide solids. Oxide and chalcogenide (S, Se, Te) or pnictide (P, As, Sb, Bi) anions differ in both size and chemical requirements, so mixed anion compounds can often be described as oxide layers separated by distinct chalcogenide or pnictide-containing slabs. The physical and chemical properties of these materials are related to the crystal structures and the chemical properties of the elements. The properties exhibited by oxide chalcogenides include semiconductor properties, for example, in LaOCuCh (Ch = chalcogenide) and derivatives, unusual magnetic properties exhibited by the class Sr2MO2Cu2-δS2 (M = Mn, Co, Ni), and redox properties exhibited by the materials Sr2MnO2Cu2m-0.5Sm+1 (m = 1 - 3) and Sr4Mn3O7.5Cu2Ch2 (Ch = S, Se). Oxide pnictides have received less recent attention, but the discovery of high-temperature superconductivity (> 40 K) in derivatives of the layered iron oxide arsenide LaOFeAs initiated a persistent wave of interest in these layered multi-anion compounds.
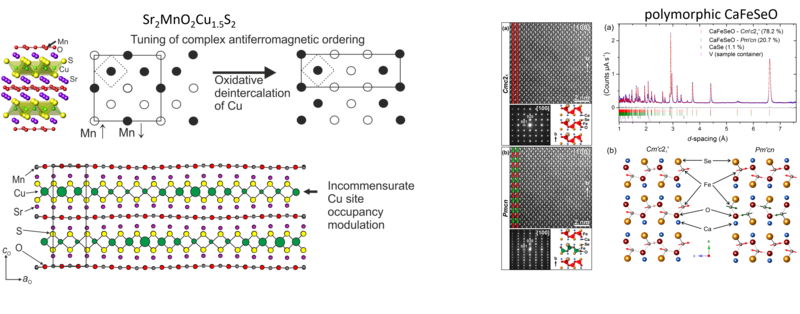
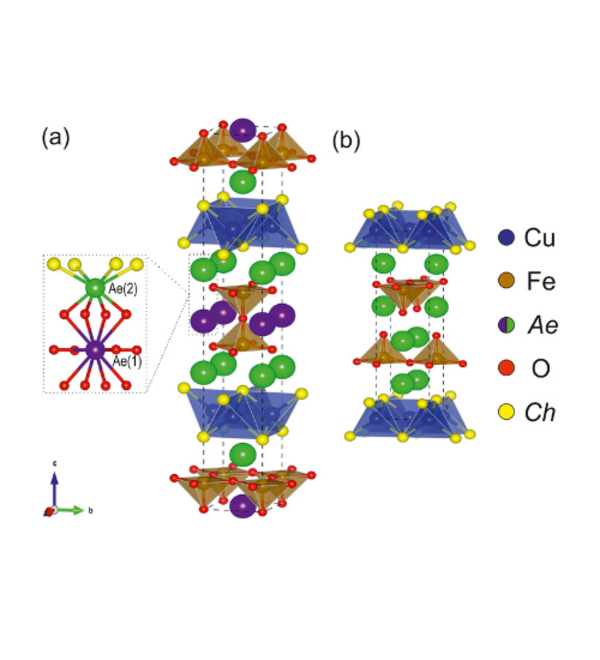
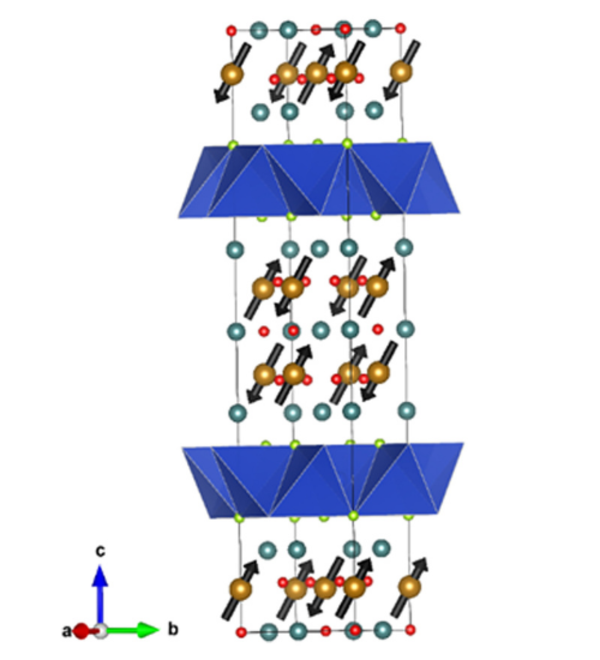
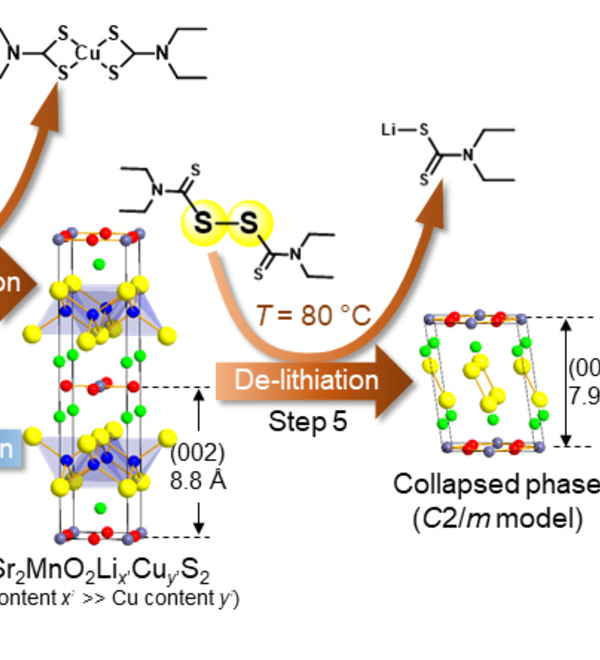
The oxide and chalcogenide or pnictide layers in these layered compounds may be considered quite electronically distinct. The degree to which this is true depends on, for example, the size of the electropositive cation A2+ in the A2MO2Cu2S2 structure, which dictates the M - S distance and hence the extent to which the transition metal M is engaged in bonding interactions with the chalcogenide anion. One other clear element of flexibility in these structures concerns the variable occupancy of the metal ion (normally Cu+ or Ag+) in the tetrahedral sites in the chalcogenide layer. We have shown that the members of the series Sr2MnO2Cu2m-δSm+1 (m = 1 -3 ) all have a very substantial copper deficiency (δ ∼ 0.5), and a range of copper deficiencies have been reported for CeOCu1-δS (0 < δ < 0.25). The implication of copper deficiency is that the transition metal or lanthanide is oxidized, and we have shown using Mn K-edge XANES measurements and magnetometry backed up by bond-valence calculations that the Mn oxidation state in Sr2MnO2Cu2m-δSm+1 (m = 1 - 3; δ ∼ 0.5) is 2.5+, which has important implications for the magnetic properties. Recently we have shown that extreme oxidation of A2MO2Cu2S2 can result in full removal of the Cu ions from the sulfide layer with anion oxidation occurring to yield disulfide [S2]2- ions, and we are currently exploring further this anion-redox chemistry.