New Nitrides
Ternary nitrides are a growing class of material and are expected to exhibit properties complementary to those of the better known oxides and chalcogenides. Binary, ternary and higher nitrides of these elements are less thermodynamically stable than the corresponding oxides as the greater energy cost associated with breaking the strong bond in N2 relative to that in O2 is not compensated for by a corresponding increase in bonding in the nitride product relative to an oxide product. The effects of this are familiar to those working in the area of nitride chemistry: nitrides are often subject to aerial oxidation or hydrolysis, although many are kinetically stable at ambient temperatures and are of increasing technological importance.
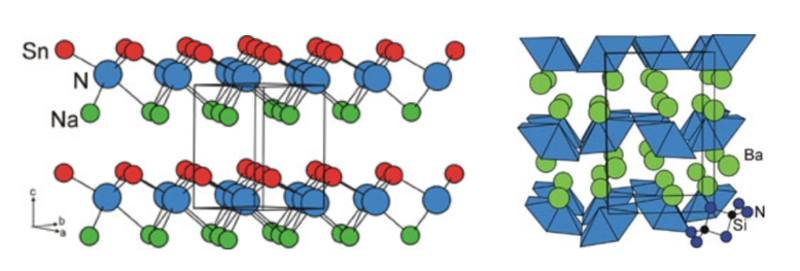
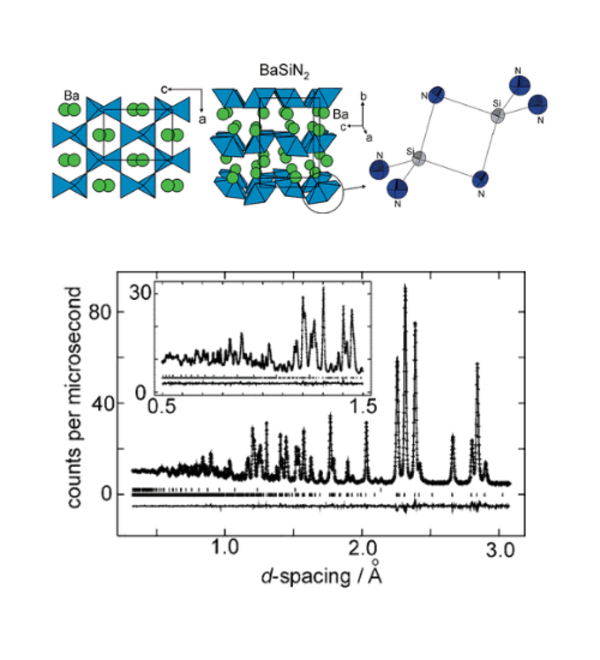
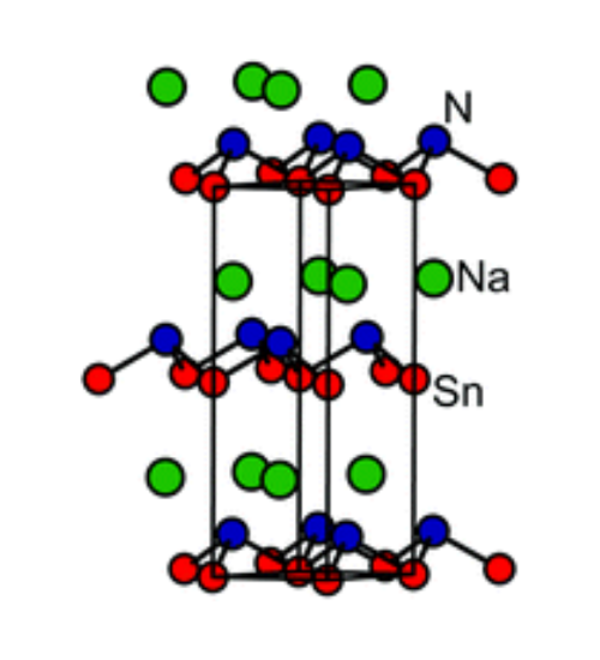
We have explored new routes to ternary nitrides including the oxidation of a highly electropositve intermetallic binary with ammonia gas. BaSiN2 was synthesised by the reaction between BaSi and flowing NH3 at the comparatively low temperature of 550 °C. This ternary nitride is quite thermally stable and may also be synthesised at much higher temperatures via the reaction between the binary nitrides under anaerobic conditions. The true value of the reaction between an intermetallic and ammonia is in the bulk synthesis of nitrides which are so thermally unstable with respect to the elements or an intermetallic that they cannot be made by routes in which a large amount of heat energy is required to overcome the activation barrier to the reaction. Our synthesis of the metastable NaSnN by reacting NaSn with ammonia exemplifies this. We are extending the exploration of new materials to include rather rare types of compound such as nitride sulfides by using synthetic ingenuity.