Exotic Superconductors
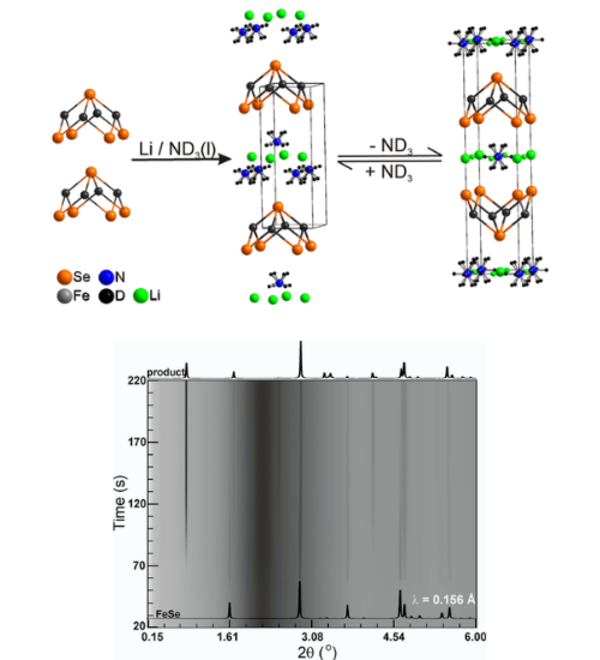
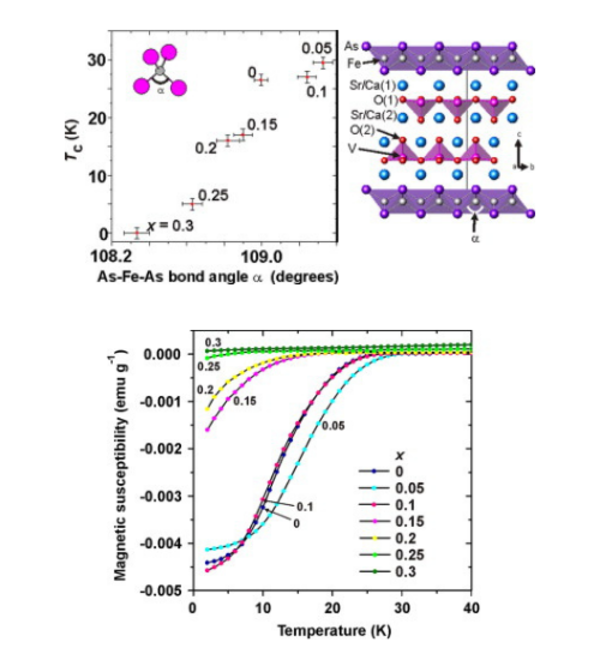
In early 2008 a new class of high temperature superconductors was discovered based on compounds containing iron arsenide layers such as LaFeAsO and BaFe2As2. Superconducting transition temperatures as high as 55 K have been confirmed in this class of material and there is abundant evidence that, rather like in the case of the cuprate superconductors, the superconductivity in these compounds cannot be explained using well-established theories of superconductivity. Our work is focused on making new compounds containing iron arsenide or iron selenide layers and investigating the link between superconductivity, composition, crystal structure and magnetism. Our early results in this area include the discovery of superconductivity in LiFeAs and the synthesis of an isostructural analogue NaFeAs which lies at the boundary between antiferromagnetism and superconductivity. Recently we have described chemical approaches to controlling the superconductivity in iron selenides using the intercalation of lithium and ammonia into iron selenide, and using hydrothermal synthesis. We are collaborating extensively with colleagues in Oxford Physics and at the Diamond Light Source, and with other colleagues around the World in understanding these exciting new materials.
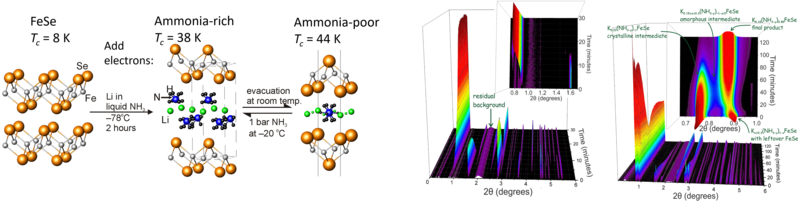
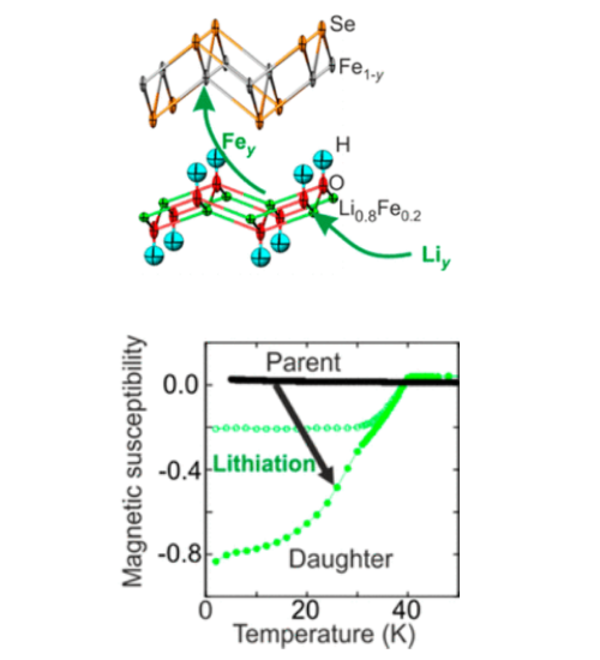
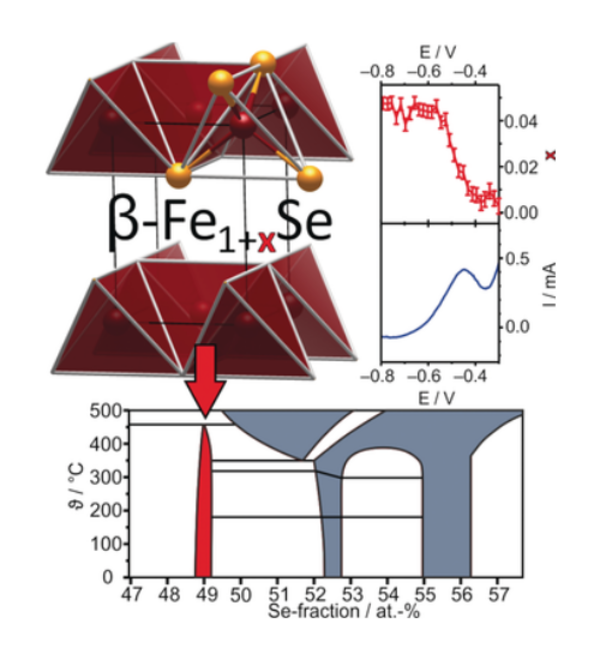
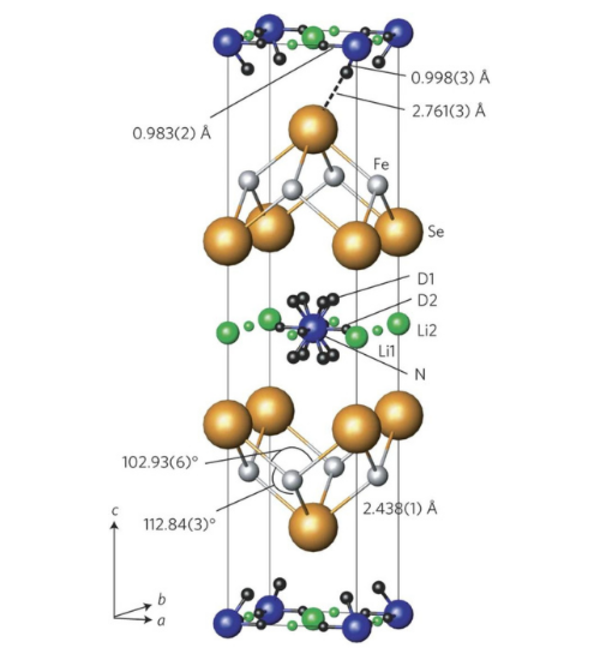
In the iron selenide area we identified the chemical factors that control superconductivity in these compounds. This included the characterisation of Lix(NH2)y(NH3)1−yFe2Se2 (x ∼ 0.6; y ∼ 0.2), with lithium ions, lithium amide and ammonia acting as the spacer layer between FeSe layers, and the control of the superconductivity in lithium iron hydroxide selenides. All these compounds exhibit superconductivity at over 40 K, much higher than in bulk FeSe. We have determined the crystal structures using neutron powder diffraction and used magnetometry and muon-spin rotation data to determine the superconducting properties. Low temperature synthesis was key to identifying these compounds which are classed as metastable and decompose at the high temperatures often used in solid state synthesis. In some cases we have used in situ methods to follow chemical or electrochemical transformations in the X-ray beam, which has enabled new phases to be discovered and which may be applicable in many other systems.